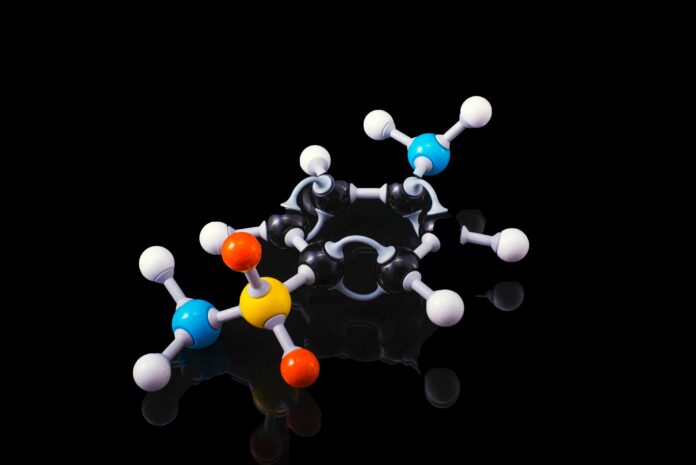
The methyl group is an essential structural unit in organic chemistry, consisting of one carbon atom bonded to three hydrogen atoms (-CH3). It plays a crucial role in a wide range of biological and chemical processes, making it one of the most important functional groups in organic compounds. Understanding the properties, reactivity, and significance of the methyl group is fundamental in various scientific disciplines, including biochemistry, pharmacology, and synthetic chemistry. In this response, I will provide you with a comprehensive overview of the methyl group, exploring its characteristics, applications, and significance in different contexts.
The methyl group, represented as -CH3, is an alkyl group derived from methane (CH4). It is a nonpolar and hydrophobic unit that is often encountered in organic molecules, such as alkanes, alkenes, alcohols, and carboxylic acids. The presence of a methyl group significantly influences the physical and chemical properties of the compounds in which it is found. Due to the high electronegativity of carbon and hydrogen, the methyl group is typically considered as a nonreactive moiety, but its chemical behavior can be altered in the presence of certain functional groups or under specific reaction conditions.
Now, let’s delve into five important aspects of the methyl group:
1. Biological Significance:
The methyl group plays a crucial role in biological systems, especially in the field of epigenetics. DNA methylation, a process in which a methyl group is added to the DNA molecule, is an epigenetic modification that can regulate gene expression. Methylation patterns can impact cellular differentiation, embryonic development, and disease states. For instance, abnormal DNA methylation patterns are associated with various diseases, including cancer. Understanding the mechanisms and implications of DNA methylation is essential for unraveling the complexities of gene regulation and disease pathology.
2. Chemical Reactivity:
While the methyl group is generally considered chemically inert, it can participate in certain reactions under appropriate conditions. For example, methyl groups can undergo substitution reactions, where one or more of the hydrogen atoms are replaced with other functional groups. This reactivity is particularly evident in organic synthesis, where methyl groups serve as versatile handles for introducing various substituents. Furthermore, methyl groups can also undergo oxidation or reduction reactions, leading to the formation of other functional groups.
3. Structural Influence:
The presence of a methyl group in an organic compound can significantly affect its physical and chemical properties. In terms of physical properties, the addition of methyl groups can increase the boiling point and decrease the solubility in polar solvents. This property is exemplified in the series of alkanes, where an increase in the number of methyl groups results in an increase in boiling point. Moreover, the steric bulk of the methyl group can influence the reactivity and selectivity of chemical reactions. For instance, the presence of methyl groups in certain positions of a molecule can hinder or enhance specific reaction pathways.
4. Methyl Donors and Biological Methylation:
Methyl groups are often utilized as “methyl donors” in various biological processes. S-adenosylmethionine (SAM) is a common methyl donor in biological systems, providing a methyl group for DNA methylation, protein methylation, and other methylation reactions. These methyl transfer reactions play critical roles in cellular metabolism, neurotransmitter synthesis, and regulation of enzyme activity. Understanding the mechanisms of biological methylation is essential for elucidating cellular processes and developing therapeutic interventions.
5. Analytical Techniques:
The methyl group also plays a vital role in analytical techniques used in chemistry and biology. Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical tool that can detect and characterize methyl groups in organic compounds. By analyzing the NMR spectra of compounds, researchers can determine molecular structure, identify compounds, and study chemical dynamics. Methyl groups are often used as diagnostic signals in NMR spectroscopy due to their distinctive chemical shifts and splitting patterns.
The methyl group, composed of one carbon and three hydrogen atoms, is a fundamental unit in organic chemistry with significant importance in various scientific disciplines. Its involvement in biological processes, chemical reactivity, structural influence, role as a methyl donor, and application in analytical techniques highlights its versatility and impact. Understanding the characteristics and behavior of the methyl group provides valuable insights into the intricacies of organic chemistry, biochemistry, and molecular biology.
In addition to its biological significance, the methyl group’s chemical reactivity is another essential aspect to consider. While it is generally considered inert, certain conditions or functional group interactions can activate the methyl group, allowing it to participate in various reactions. Substitution reactions, where the hydrogen atoms of the methyl group are replaced with other functional groups, can occur under specific circumstances. This reactivity is particularly valuable in organic synthesis, where methyl groups serve as versatile handles for introducing desired substituents into target molecules. Furthermore, methyl groups can undergo oxidation or reduction reactions, leading to the formation of other functional groups and expanding their utility in synthetic chemistry.
The structural influence of the methyl group cannot be understated. Its presence in an organic compound significantly affects its physical and chemical properties. The addition of methyl groups can increase the boiling point and decrease solubility in polar solvents. This property is evident in the series of alkanes, where an increase in the number of methyl groups corresponds to an increase in boiling point. Moreover, the steric bulk of the methyl group can impact the reactivity and selectivity of chemical reactions. In certain positions within a molecule, methyl groups can hinder or enhance specific reaction pathways, providing control over reaction outcomes and enabling the synthesis of complex molecules with desired stereochemistry.
Methyl groups also have a role as methyl donors in various biological processes. S-adenosylmethionine (SAM), a key molecule in cellular metabolism, acts as a methyl donor by transferring its methyl group to various acceptor molecules. DNA methylation, protein methylation, and other methylation reactions rely on the availability of methyl groups provided by SAM. These methylation reactions play critical roles in gene regulation, cellular signaling, and enzymatic activity. Understanding the mechanisms of biological methylation and the significance of methyl donors like SAM is vital for elucidating fundamental cellular processes and developing therapeutic interventions.
Moreover, methyl groups find application in analytical techniques used in chemistry and biology. Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful tool for characterizing organic compounds and elucidating their molecular structures. Methyl groups often serve as diagnostic signals in NMR spectroscopy due to their distinctive chemical shifts and splitting patterns. By analyzing the NMR spectra of compounds, researchers can determine molecular connectivity, identify compounds, and study dynamic processes. The ability to detect and analyze methyl groups in NMR spectroscopy enhances our understanding of chemical structures and aids in the development of new compounds and materials.
In summary, the methyl group is a fundamental unit in organic chemistry with profound implications in various scientific domains. Its biological significance, chemical reactivity, structural influence, role as a methyl donor, and application in analytical techniques highlight its versatility and importance. By comprehending the properties and behaviors of the methyl group, scientists can advance their understanding of organic chemistry, biochemistry, and molecular biology, leading to discoveries and innovations that benefit numerous fields.